The Environmental Mineralogy Group (EMG) is launching its inaugural photo competition with the hope of showcasing the best environmental mineralogy has to offer.
As a means of celebrating how interesting and engaging environmental mineralogy can be, we would like to welcome entries to the competition in any area where environmental mineralogy is showcased, be that out in the field, in the laboratory, or even under the (electron) microscope.
Submitting to the competition is easy, simply send your entry to the email address below with a description of your photo detailing why your entry is interesting. We welcome entries for all stages of careers in environmental mineralogy; it doesn’t matter if you’re a student or an established expert, we want to see your photos!
Monetary prizes will be awarded in a number of categories and the winning photos will be displayed on the EMG website and in the EMG / MinSoc Newsletters.
The deadline for submissions is Friday 28th February.. Submit your entries (or any queries you may have) here: emg@minersoc.org
Here are a couple of examples to get you started:
Example 1:
A profile of intertidal flat sediments in the German Wadden Sea. The image shows several redox features including the precipitation of iron (oxyhydr)oxides (orange/brown) in lugworm burrows formed as a result of oxygen in the burrows oxidising reduced iron in the otherwise grey sediment. The sediment becomes darker/black with depth indicating the formation of a redox profile.
The sediments likely undergo cycles of reduction during tidal flooding and oxidation during the ebb tide, causing the iron minerals in the sediment to dynamically precipitate and dissolve. We are researching the implications of these iron mineral transformations for the cycles of nutrients and contaminants.

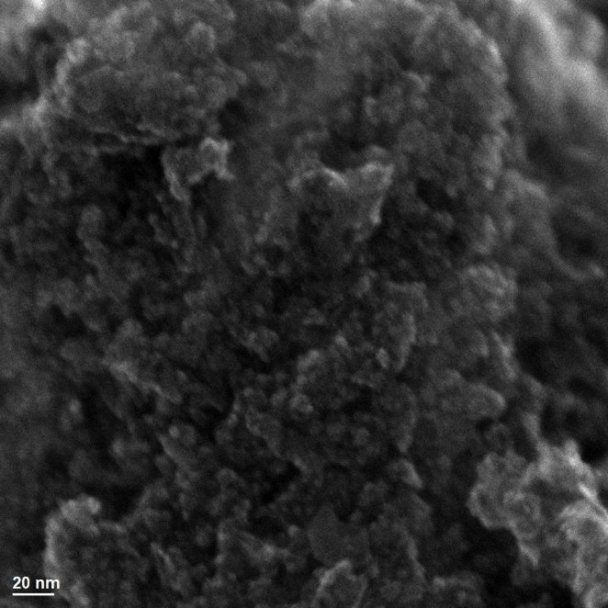
Example 2:
SEM image of ferrihydrite. While this image may look unimpressive with no discernible crystalline structures visible at the nanometre scale, this highlights the importance of ferrihydrite (a short range ordered iron oxyhydroxide) in the environment. Due to its short range ordered nature, ferrihydrite has an extremely high specific surface area and is therefore an important sorbent for contaminants and nutrients.